A solution of a strong alkali at concentration 1 M (1 mol/L) has a pH of 14. Thus, in most problems that arise pH values lie mostly in the range 0 to 14, though negative pH values and values above 14 are entirely possible. Weak acid/base. Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a bit more complicated.. pH + pOH = 14 (Eq. 3) This relationship can be used to convert between pH and pOH . In combination with Eq. 1a/b and Eq. 2a/b, we can always relate pOH and/or pH to [ OH −] and [ H +] . For a derivation of this equation, check out the article on the autoionization of water.
The pH of a solution obtained by mixing 100ml of 0.2 M CH3COOH with 100 ml of 0.2 M NaOH will be
/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)
How to Prepare a Sodium Hydroxide or NaOH Solution

Calculate Ph of Buffer After Adding Naoh

How To Calculate Ph From Molarity Of Naoh

Determine the pH of 102 M NaOH solution. Brainly.in

0.5 M NaOH Solution YouTube
Naoh

Calculate Ph of 105M of naoh Brainly.in

AcidBase Titrations General Chemistry
[Solved] 1.Calculate the pH of a solution that is 0.5 M in ammonium chloride... Course Hero
Calculate pH of 0.01M solutions of NaOH

Calculate the pH of 0.05M NaOH solution Brainly.in

Ph as a Function of Volume of NaOH scatter chart made by Asmaley plotly
Как определить ph раствора дома 84 фото
[Solved] Calculate the pH of the solution resulting from the addition of... Course Hero

PH DE HIDRÓXIDO DE SODIO AÑADIENDO GRAMOS DE NaOH CAMBIANDO LA CONCENTRACIÓN DE LA SOLUCIÓN
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube

Calculate the PH of 0.0001 molar solution of NaOH Brainly.in

A Amostra De 100 Ml De Naoh EDUCA
21. Calculate the PH of a solution which contain 9.9 ml 1 M HCL and 100ml of 0.1 M NaOH
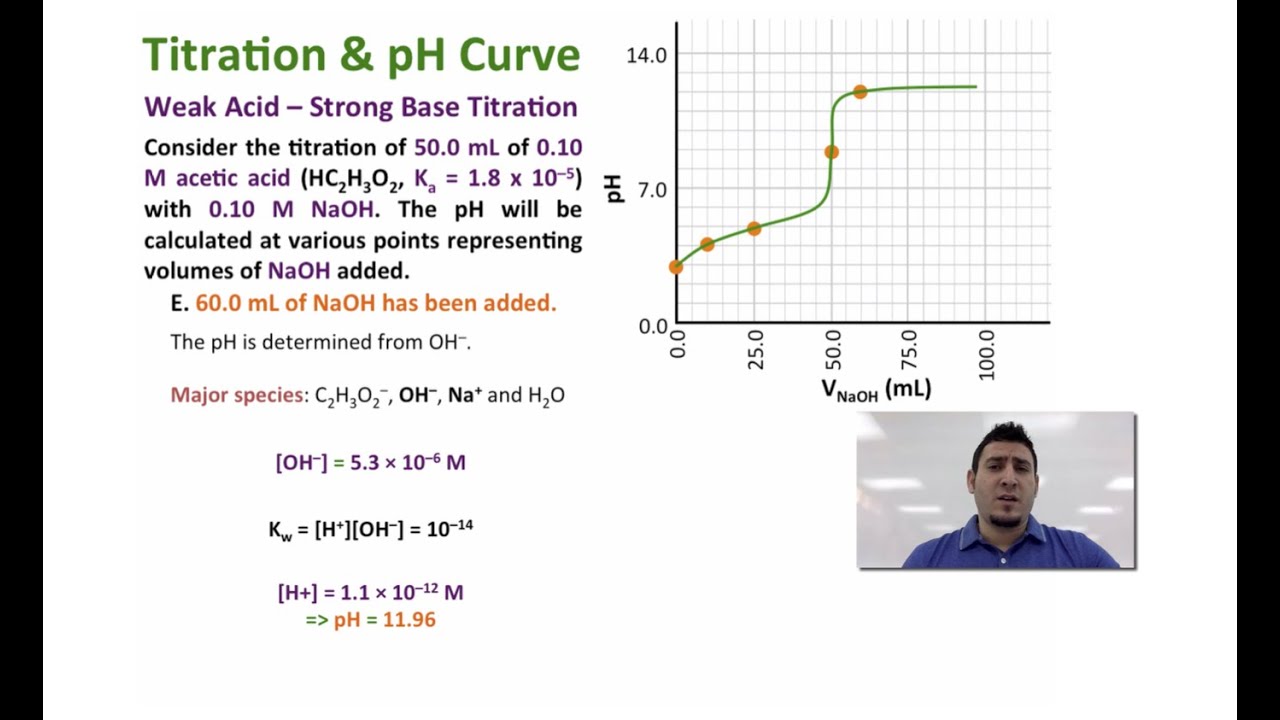
Let's assume that the concentration of hydrogen ions is equal to 0.0001 mol/L. Calculate pH by using the pH to H⁺ formula: \qquad \small\rm pH = -log (0.0001) = 4 pH = −log(0.0001) = 4. Now, you can also easily determine pOH and a concentration of hydroxide ions using the formulas:. (a) As 0.200 M \(NaOH\) is slowly added to 50.0 mL of 0.100 M acetic acid, the pH increases slowly at first, then increases rapidly as the equivalence point is approached, and then again increases more slowly. The corresponding curve for the titration of 50.0 mL of 0.100 M HCl with 0.200 M \(NaOH\) is shown as a dashed line.